Abstract
Background: It is very well known that information provided by clinical records and organized system platforms (registries) offer a holistic view of MDS patients' characteristics and therapeutic aspects in real-life. The primary objective of this study was to analyze data from a novel MDS Latin-American Registry.
Methods: The registry started in April 2022. From April to July, patients with MDS or CMML diagnosed since January 2014 were reported from Argentina, Brazil, Colombia, Mexico, and Uruguay. The Research Electronic Data Capture (REDCap) platform was used, consisting of 298 fields to describe patient demographics; their biological characteristics; clinical manifestations; comorbidities; laboratory features; bone marrow studies; risk classification; treatments, and outcomes. According to the Ethics Committee, all subjects had to sign an inform consent. Descriptive statistics were used to summarize demographic, and clinical data, as well as treatment characteristics - overall and by risk groups.
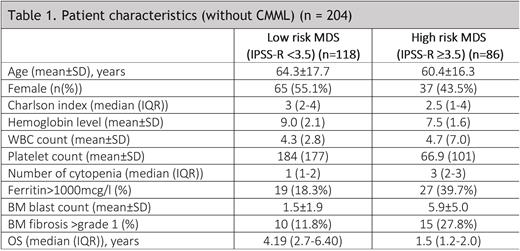
Results: A total of 231 patients were included in this analysis. The mean age was 62.8 ± 16.9 years and the male to female ratio was 1.1:1. Baseline characteristics are described in Table 1. According to WHO's 2016 classification, the following categories were reported: MDS-SLD 4.4%; MDS-RS 1.3%; MDS-MLD 34.5%; MDS-RS-MLD 4.4%; MDS-EB 24.9%; MDS with isolated del(5q) 2.2%; unclassifiable MDS 0.4%; therapy-related MDS 7.0%; CMML 10.9%; MDS-U 1.3%; MDS/MPN-RS-T 0.4%; aCML 0.4%; other 7.9%. The IPSS-R was: 3.4% Very Low; 62.3% Low; 17.9% Intermediate; 10.1% high-risk; and 6.3% very high-risk. The majority of patients had de novo MDS (95.2%), and 8.6% had hypoplastic MDS. The mean BM blast count was 3.5±4.3%. Iron staining was performed in 28.1%. BM biopsies were performed at the moment of diagnosis in 77.5% of cases, reported as hypercellular in 90.0% of them and with fibrosis (> grade 1) in 32%. Immunohistochemistry was performed in 53.7% and flow cytometry in 47.8% of patients. Cytogenetics was performed in 93.9% and FISH in 26.9%. Abnormal karyotype was detected in 38.2%. An NGS panel was performed in 35 (15.2%) patients.
Low-risk MDS accounts for the majority of patients in our registry (58%). Erythropoietin was given in 73 (69.5%) and growth factor therapy in 13 (11%) patients. Red blood cell transfusion was given to 59 (50%) patients. 31 (26%) of them received second-line treatment: 29 with HMA, and 2 with lenalidomide. 5 (4.2%) underwent allogenic transplant. 16 (13.7%) progressed to AML. The median overall survival was 4.19 (2.7-6.40) years.
High-risk MDS was reported in 42% of patients. Regarding treatment, 69 (80.2%) received HMA (AZA=65 and DEC=4), 4 (4.6%) chemotherapy, and 8 (9.3%) of them were administered a venetoclax-based therapy. Fifteen (17.4%) were transplanted and 46 (53.4%) progressed to AML. The median overall survival was 1.5 (1.2-2.0) years.
Conclusions: This novel and international registry showed preliminary MDS data from Latin-America. Our results revealed younger age at diagnosis, high number of patients undergoing allogenic transplant and OS according to literature. The expansion of this registry certainly improves our clinical practice.
Disclosures
Gomez-De Leon:AbbVie: Honoraria, Other: advisory board. Iastrebner:Abbvie: Honoraria, Other: Advisory Board, Speakers Bureau; BMS: Honoraria, Other: Advisory Board, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal